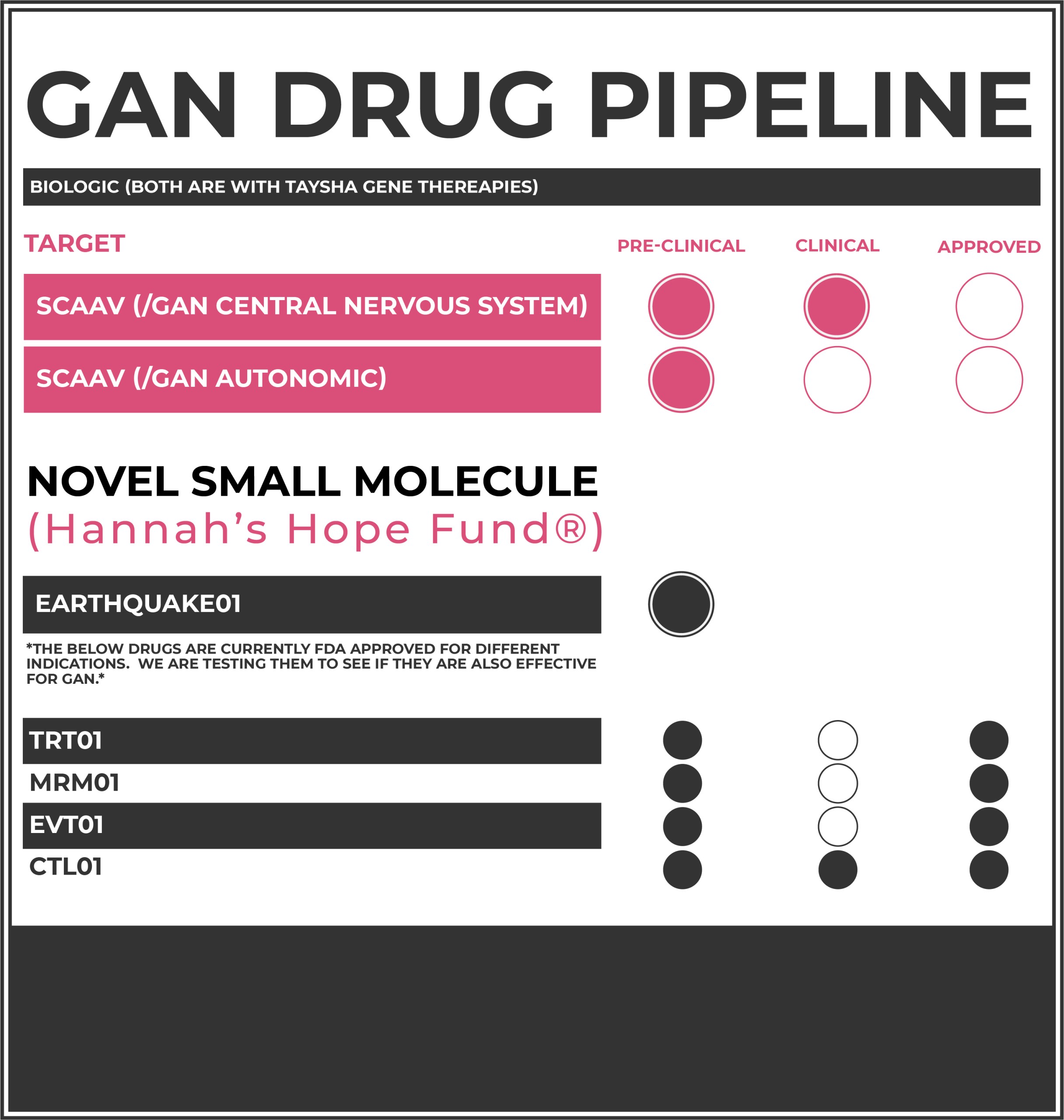
What is the Status of Research on scAAV9/GAN?
What is the scAAV9/GAN Investigational New Drug?
Hannah’s Hope Fund had monies available to fund everything except the actual clinical trials. Lori approached the National Institutes of Health (NIH) and Dr. Carsten Bonnemann agreed to run a clinical study out of the NIH Clinical Center in Bethesda, MD.
In May of 2015, the first patient in the world to receive a therapeutic gene to the spinal cord via spinal fluid was a patient with Giant Axonal Neuropathy! Yes, this was a first-in-human made possible by the thousands of people supporting Hannah’s Hope Fund since Hannah’s diagnosis in 2008!
We are praying the government reinstated the Pediatric Priority Review Program (PPRV) or else there’s no incentive for biotechs to invest in rare disease. Incentives MUST be put in place in order for the treatments parents like Lori Sames are working tirelessly to provide for generations to come.
HHF is opening a secondary injections site for the central nervous system AAV9 gene therapy that’s still ongoing at the NIH since 2015. We hope to move very quickly to get more patients injected.
EXCITING NEWS! HHF, in collaboration with UTSW, in Dallas has developed another first-in-human gene therapy. This time, we are targeting the autonomic nervous system by directly injecting the AAV9/GAN gene therapy vector (drug) into the left vagus nerve. This injection aims to target speech, swallowing and involuntary breathing.
This novel route of administration has broad implications for more common disorders like ALS and Parkinson’s Disease.
AAV9/GAN gene therapy clinical trial identifier: NCT02362438
Research
The Adeno Associated Virus serotype 9 (AAV9) is a benign viral vector, meaning it’s a virus that can’t cause disease in humans that’s specially designed to deliver gene therapy. The AAV9/GAN virus is injected into the cerebral spinal fluid of patients through a needle into the spine called a lumbar puncture (intrathecal route of administration). The virus carries the healthy gene through the central nervous system and ‘infects’ nerve cells and some glial cells with the healthy copy of the Giant Axonal Neuropathy (GAN) gene. These new genes will direct cells to correctly make the protein that is missing in GAN patients. Because gigaxonin stays inside cells, it’s important to infect as many cells as possible with a healthy GAN gene because gigaxonin isn’t taken up by neighboring cells.
The higher the dose of AAV9 viral particles, the greater the spread of the virus which results in more nerve cells being infected with healthy (non-mutated) GAN genes.
The AAV machinery enables the cells to express gigaxonin, the protein byproduct of the GAN gene.
New Frontiers in scAAV9/GAN Research
Hannah’s Hope Fund, in collaboration with Dr. Rachel Bailey and Dr. Gray, University of Texas Southwestern (UTSW) began funding a GAN gene transfer program targeting the autonomic nervous system, the nerves that control involuntary processes. Taysha learned of this work through its collaboration with UTSW and was eager to provide funding for future studies testing the autonomic nervous system approach.
Thus far, the approach has been tested in three different animal models and the surgical protocol for testing has been developed. Once animal toxicology studies are conducted to investigate the safety of the approach, a clinical study should rapidly begin, baring no ill effects are identified.
The goal would be to eventually give both the lumbar (intrathecal) and autonomic injections simultaneously. However, the autonomic AAV9 trial must begin on Giant Axonal Neuropathy (GAN) patients who have already received intrathecal AAV9/GAN to establish safety and efficacy. Initial data indicate AAV9/GAN will not be blocked by the immune system in patients who have already received AAV9/GAN intrathecally to the CNS.
Novel Compounds for GAN
Because GAN is a multi-system disease impacting every cell in the body, it’s critical we develop a cocktail of drugs to help GAN patients live their best lives.
HHF has a novel compound we refer to as “earthquake”. The protein not working in the cells of GAN patients is called gigaxonin. Gigaxonin’s role is to flag other proteins in cells to be broken down when they are done doing their jobs. Without enough functional gigaxonin protein, these proteins, called intermediate filaments, build up inside nerve cells, causing the axons to swell and damage cells. The earthquake compound takes down the scaffolding the intermediate filaments are building upon, perhaps allowing them to be cleared by another mechanism.
Global Registry for Inherited Neuropathy (GRIN)
In 2013, Hannah’s Hope Fund launched a Global Registry for Inherited Neuropathy (GRIN) in collaboration with the Hereditary Neuropathy Foundation. The goal of the registry is to identify patients with various forms of inherited neuropathy, assist them in receiving a genetic diagnosis, and enable them to engage in research for their disease. GRIN is a valuable tool to engage industry, as we are able to tell a drug company how many patients have the disease for a specific drug indication, how many patients said they would be willing to provide tissue samples to drive translation research and how many patients said they would be willing to consider volunteering for experimental trials.
Unless the industry knows they have a solid, engaged audience, they will not invest in drug development for rare diseases. The vast majority of inherited neuropathies are considered ultra-rare, impacting less than 600 US patients. Through GRIN, we hope to find more GAN patients who have been clinically diagnosed with Charcot-Marie-Tooth Disease type 2 (axonopathy).
GAN Natural History Study
Hannah’s Hope Fund began funding a natural history study on GAN at Columbia, in NY City, back in 2012. The study transitioned to that National Institutes of Health (NIH), under Principle Investigators, Dr. Carsten Bonnemann and Dr. Diana Bharucha.
A natural history study is a complete review of disease progression over time, with the goal of arriving at clinical endpoints to evaluate the safety and effectiveness of investigational new drugs.
Drug Repurposing:
There are a few FDA approved drugs that showed to be efficacious in cell models of GAN (neurons and astrocytes) during a high-throughput drug screen of the FDA Approved Drug Library. We are currently designing Giant Axonal Neuropathy mouse studies to test the drugs, in vivo, to see if they rescue pathology identified in the GAN mouse model.